Theme 4
Theme 4: Material Synthesis Triggered by the Transformation of Amorphous Precursors
Biomineralisation often occurs via the precipitation of amorphous or poorly ordered precursor phases which are deposited and moulded before the final mineral is formed. This allows the organism to control nucleation and growth processes with precision, to shape the mineral phase into a desired morphology and to control the crystallographic orientation. In this sub-project we are controlling the transformation of amorphous materials in order to generate large single crystals of different polymorphs.
4.1 A Two-Step Strategy for Preparing Large Single Crystal Calcite Films
The use of amorphous calcium carbonate (ACC) in biomineralization processes affords organisms outstanding control over the formation of calcite and aragonite biominerals. Essential to this strategy is that the ACC is maintained within confined volumes. A pseudomorphic transformation can then occur in the absence of bulk water, and organisms can independently control nucleation and growth. However, comparable control has proven hard to achieve in synthetic systems. We here demonstrate a straightforward, two-step method for controlling the crystallization of ACC thin films, where nucleation was first triggered at a single site using a heated probe that triggers instantaneous nucleation, and then sustaining growth by incubating the film at a lower temperature. By independently controlling nucleation and growth we can readily generate millimeter-scale calcite crystals when and where we want, create morphologies ranging from discs, to squares to serpentine strips, and create patterned arrays of crystals. We also study the mechanism of crystallization using in situ TEM and demonstrate that the growth of calcite from an amorphous precursor is associated with a high activation energy. Propagation of the growth front during pseudomorphic transformation of ACC at room temperature is therefore likely to occur via local dissolution/ reprecipitation.
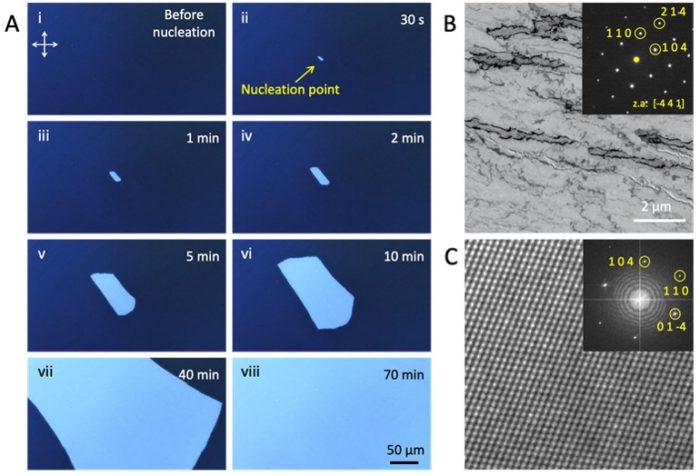
Figure. Production of large single crystals of calcite, where nucleation was triggered at a single site with a heating probe, and the film was then uniformly heated. (A) Sequential POM micrographs showing (i) the initial ACC precursor film, (ii) the nucleation point, where the image was recorded after 30 sec, (iii-viii) Continued transformation of the ACC films. (B) Bright field TEM image and corresponding SAED pattern (inset) of the product single-crystal calcite film. (C) HRTEM image and corresponding FFT image.
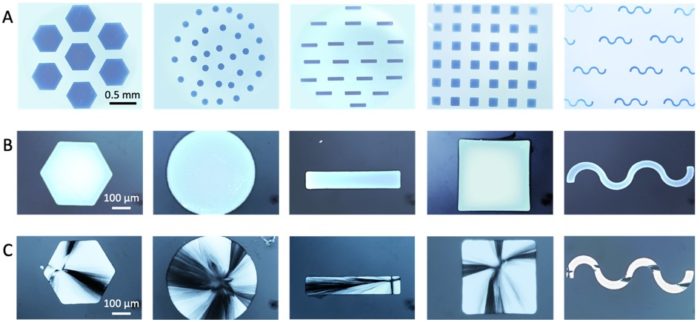
Figure. Creating large single crystals with arbitrary morphologies. OM images of (A) large ACC films with shapes including hexagons, circles, stripes, squares and serpentine stripes, (B) large single crystals of calcite formed by the two-step control method with nucleation triggered with local heating and then growth sustained, and (C) polycrystalline calcite crystals formed at ambient conditions.
4.2 The Magnesium Content of Amorphous Calcium Carbonate Thin Films Determines the Polymorph in Solid-State Transformation
Amorphous calcium carbonate (ACC) is a common precursor to crystalline calcium carbonate, and is of particular importance in biomineralization, where its crystallization in privileged environments ensures a pseudomorphic transformation. Organisms regulate this process using organic molecules and magnesium ions to create both calcite and aragonite biominerals. However, although pseudomorphic transformation of ACC to calcite is readily achieved in synthetic systems, there are very few examples of transformation to aragonite. Here, we use heat to induce a pseudomorphic transformation of ACC thin films containing magnesium, and show that the structure, composition and polymorph of the product films are determined by the magnesium content of the ACC and the heating regime employed. Films with spherulitic structures form at room temperature while isothermal heating generates a mosaic of large single crystals. Notably, the annealed films can be tuned from calcite, to pure aragonite, through low and high magnesium calcite, and ultimately dolomite by increasing the magnesium content of the ACC. Deposition of the ACC films on suitable templates then allows us to create aragonite single crystals with arbitrary morphologies. These experiments therefore suggest that organisms may control calcium carbonate polymorph by tuning the composition, and in particular the magnesium content of the precursor ACC.
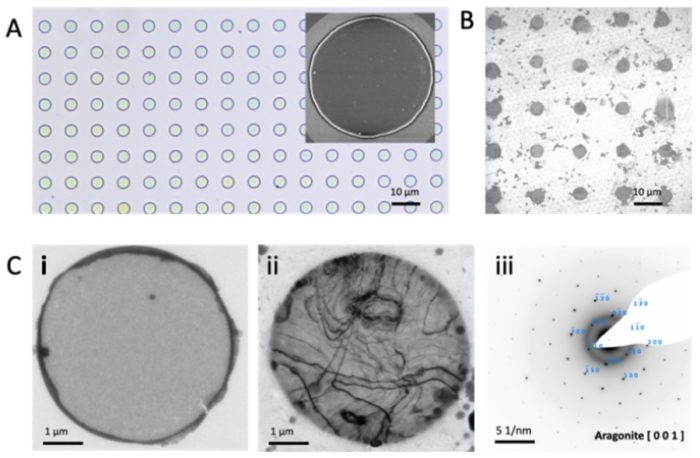
Figure. Patterned ACC and crystallization to aragonite. (A) OM and (inset) SEM images of arrays of ACC discs with diameters of 5 µm on silicon wafers. TEM Bright-field images of (B) and (Ci) circular ACC films patterned on TEM grids and (Cii) aragonite formed on heating. (Ciii) SAED pattern of the aragonite shown in (ii).
